Allyltrimethylsilane 烯丙基三甲基硅烷
规格: 98%
CAS:
762-72-1
产品编号: H65036
MDL:
MFCD00008635
品牌: INFI
简介
有机硅烷类化合物主要应用在有机合成及有机硅材料领域。在有机合成中,硅烷作为羟基 保护基大量存在,如三甲基氯硅烷,TBSCI,有时作为一个合成子载体存在,如乙烯基三甲基 硅烷,烯丙基三甲基硅烷,六甲基二硅硫烷等,从而实现相应的官能团化反应。有机硅材料领域有硅烷偶联剂、硅油(硅脂、硅乳液、硅表面活性剂)、高温硫化硅橡胶、液体硅橡胶、硅 树脂、封端剂,含硅复合共聚物等。
应用介绍及参考文献
1.如乙烯基三甲基硅烷作为一个“乙烯”合成子,用于3+2环加成反应,应用于有机合成过程中。

图片来源于期刊:Vinyltrimethylsilane, Glenn J. Fegley Gerald L. Larson, Encyclopedia of Reagents for Organic Synthesis, 2008.
2.如烯丙基三甲基硅烷,作为一个烯丙基化试剂,应用于艾日布林或者前列素类似物的合成中。
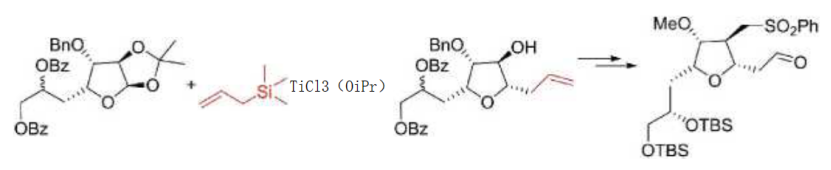
图片来源于期刊:W020050118565, WO2019102490

图片来源于期刊:Gary A. Sulikowski et al Org. Lett. 2019, 21, 10048-10051
3.作为聚合引发剂,或者烯姪共聚物,参与烯姪聚合,形成功能性有机硅材料,如耐水性硅胶 粘合剂,修饰聚氟乙烯等
技术优势
产品纯度高,具有百公斤生产能力
| Chemical Name | Allyltrimethylsilane |
|---|---|
| Synonym | Allyltrimethylsilane {LY} Allyltrimethylsilane allyltrimethylsilane {} {LY} Allyltrimethylsilane {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} { {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethylsilane {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} Allyltrimethyl |
| MDL Number | MFCD00008635 |
| CAS Number | 762-72-1 |
| EC Number | 212-104-5 |
| Beilstein Registry Number | 906755 |
| PubChem Substance ID | 69808 |
| Reaxys-RN | 906755 |
| Chemical Name Translation | 烯丙基三甲基硅烷 |
| LabNetwork Molecule ID | LN00008514 |
| InChI | InChI=1S/C6H14Si/c1-5-6-7(2,3)4/h5H,1,6H2,2-4H3 |
| Canonical SMILES | C[Si](C)(C)CC=C |
| Signal word | Danger |
|---|---|
| WGK Germany | 3 |
| Safety Statements | |
| |
| Risk Statements | |
| |
| Precautionary statements | |
| |
| Packing Group | II |
| UN Number | 1993 UN 1993 3/PG 2 |
| Hazard statements | |
| |
| Personal Protective Equipment | Eyeshields, Faceshields, full-face respirator (US), Gloves, multi-purpose combination respirator cartridge (US), type ABEK (EN14387) respirator filter |
| Hazard Codes | 3 F;Xi |
| Hazard Class | 3 |
| Storage condition | 2-8°C, stored under nitrogen {LY} 2-8°C, stored under nitrogen {} {LY} 2-8°C, stored under nitrogen {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {} {} {} {} {LY} 2-8°C, stored under nitrogen {} {} {} {} {} {} {} {} {} {} {} {} {} {} { |
化学属性
| Mol. Formula | C6H14Si |
|---|---|
| Mol. Weight | 114.26 |
| Density | 0.699 |
| Refractive index | 1.4060 to 1.4080 |
| Flash Point | 45 °F |
| TSCA | Yes |
| Boiling Point | 84-88 °C |
| Solubility | 不溶 |
| Appearance | 无色液体。沸点44℃(2.4kPa),相对密度1.1628(20/4℃),折光率1.4675(20℃)。能与有机溶剂混合,不溶于水。 |
| Melting Point | N/A |
*以上化合物性质及应用等信息仅供参考


